The Alzheimer's Disease Research Center at USC
USC ADRC
Welcome to the University of Southern California Alzheimer’s Disease Research Center (USC ADRC), a leader in the fight against Alzheimer’s disease and related disorders (ADRD).
Our mission is to advance research, enhance patient care, and train the next generation of scientists. This platform is a comprehensive resource for individuals seeking opportunities to participate in studies and clinical trials while providing healthcare providers with valuable referral tools.
ADRC Research Studies
Vascular and Genetic Risk Factors for Alzheimer’s Disease II (PPG II)
The purpose of this study is to learn how genetic risk factors and changes in the brain’s vascular system may be related to an increased risk for developing Alzheimer’s disease. This study will look at biological markers for Alzheimer’s disease risk found in the blood and spinal fluid (CSF) and changes in the brain’s structure over time. We hope that the knowledge gained from this study will help us to detect the early development of Alzheimer’s disease and possibly identify new ways to treat dementia.
English- or Spanish-speaking men and women of 45 years or older are eligible for this study. Participants will receive medical examinations, tests of memory and daily function, MRI brain scans, blood tests, and a test of spinal fluid. Study participants receive a small financial compensation for visits to USC.
Read more about joining the PPG study here: English | Spanish
Please call Nadine Diaz, DSW, MSW at (213) 821-7158 for more information.
Contact to learn more: Nadine.Diaz@med.usc.edu
Vascular Cohort Study (VCD) [Includes VCD Pilot, VCD, VCS-II]
For many years, the central scientific theme of the USC ADRC has focused on vascular contributions to cognitive impairment and Alzheimer's disease (AD). Epidemiologic studies have demonstrated that traditional cardiovascular risk factors, such as hypertension and diabetes mellitus, are linked to increased risk of AD, especially among under-represented ethnic groups. The Vascular Cohort Study (VCD) is a longitudinal study directly funded by the ADRC that interrogates the health of the neurovascular unit (NVU) using novel imaging and CSF biomarkers. Using dynamic contrast enhancement (DCE)-MRI, our Imaging Core has developed a novel Ktrans measure of permeability of the blood brain barrier (BBB), one of the essential functions of the NVU. Using the Meso Scale Discovery Platform, the Biomarker Core has developed a new panel of vascular, BBB, and NVU biomarkers including cell-specific markers of pericyte, neuronal, and astroglial injury, inflammatory biomarkers, as well as AD standard Aβ and tau biomarkers that can be obtained using small quantities (0.5 ml) of CSF. Criteria for participant enrollment include age > 60 years, no or mild cognitive impairment, and presence of low, medium or high cardiovascular risk based on blood pressure, lipids, and glucose. Participants are followed annually by the Clinical Core using the NACC Uniform Data Set.
Contact to learn more: Helena.Chui@med.usc.edu
Biomarkers of ABCA1 mediated functions in Alzheimer’s (VCSGT)
VCSGT is a longitudinal study aimed at investigating the impact of diabetes on brain connectivity. By tracking participants over time, the study seeks to understand how diabetes influences the brain’s neural networks and contributes to cognitive changes. This research could provide valuable insights into the relationship between diabetes and brain health, potentially guiding future prevention and treatment strategies.
Contact to learn more: hyassine@usc.edu
Diverse VCID (DVCID)
This study aims to identify the extent and characteristics of white matter injury that influence cognitive and health outcomes. Based on the findings, the ultimate goal is to increase the understanding of precision medical management and planning needed by patients with white matter lesions.
Contact to learn more: cdecarli@ucdavis.edu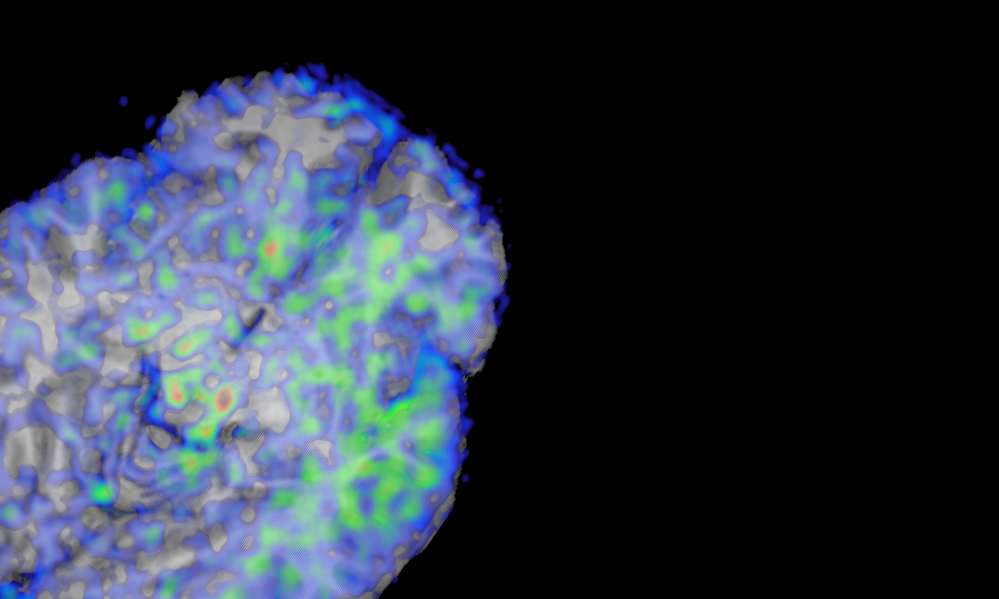
Model-based cerebrovascular markers (DVR)
Researchers have found that people with mild cognitive impairment and mild Alzheimer’s disease show different dynamic responses to changes in blood carbon dioxide compared to healthy people. The DVR project studies how changes in the dynamic regulation of brain blood flow and cortical oxygenation (called together cerebral perfusion) relate to cognitive function and memory impairment.
Please call Nadine Diaz, DSW, MSW at (213) 821-7158 for more information.
Contact to learn more: Nadine.Diaz@med.usc.edu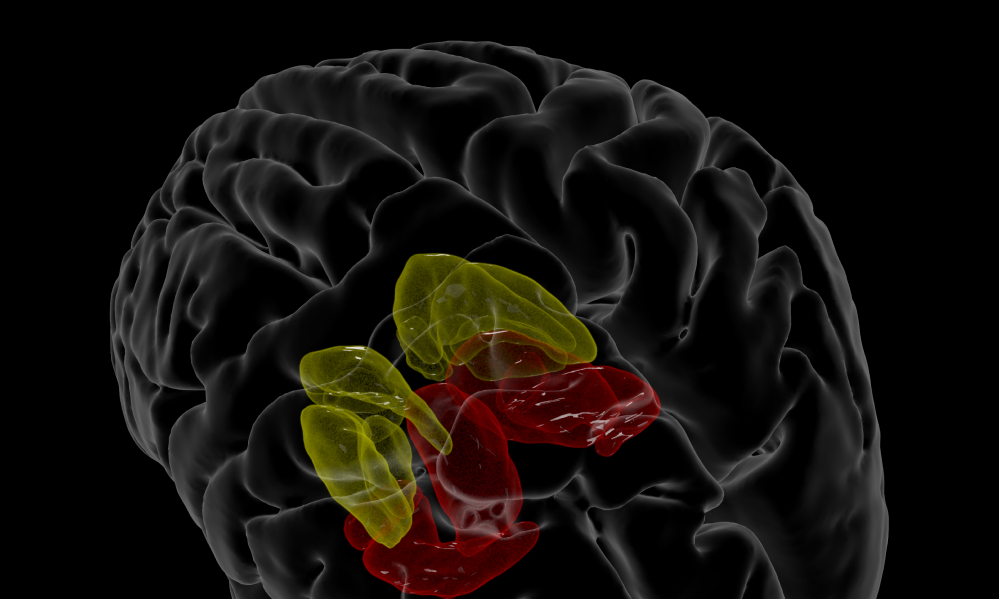
DHA Delivery to Brain Pilot Study (DHA2BRP)
Observational studies suggest a link between DHA and cognitive health, but randomized control trials show conflicting results. The DHA2BRP study investigates the relationship between DHA intake and cognitive function, particularly in individuals with the APOE e4 gene variant.
Contact to learn more: hyassine@usc.edu
Standardized Centralized Alzheimer’s & Related Dementias Neuroimaging (SCAN)
Researchers at the NIA-funded ADRCs have been collecting different types of PET and MR images using various acquisition methods for many years. Because of this, image data could not be easily combined across ADRCs, resulting in lost opportunities for scientific collaboration. The goal of SCAN is to standardize PET and MRI data collection from across the ADRC Program so that it can be combined and shared with researchers worldwide via the NACC Data Platform.
Contact to learn more: Helena.Chui@med.usc.edu